
Chiral Catalysts for Pd0‐Catalyzed Enantioselective C−H Activation - Vyhivskyi - - Chemistry – A European Journal - Wiley Online Library

Synthesis, characterization and catalytic activity of PEPPSI-type palladium–NHC complexes - ScienceDirect

Understanding Palladium Acetate from a User Perspective - Carole - 2016 - Chemistry – A European Journal - Wiley Online Library

Transition metal-catalyzed cross-coupling methodologies for the engineering of small molecules with applications in organic electronics and photovoltaics - ScienceDirect

Auxiliary-assisted palladium-catalyzed arylation and alkylation of sp2 and sp3 carbon-hydrogen bonds. - Abstract - Europe PMC
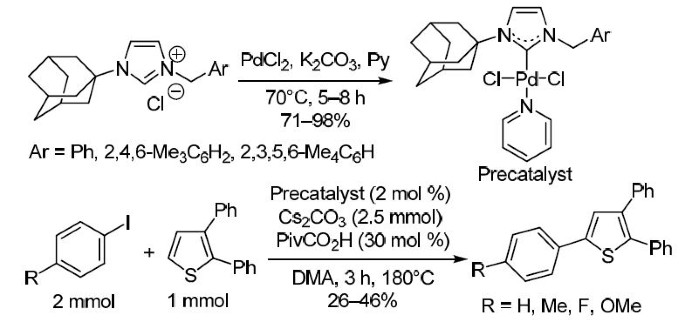
Adamantanyl-substituted PEPPSI-type palladium(II) N-heterocyclic carbene complexes: synthesis and catalytic application for CH activation of substituted thiophenes | SpringerLink
![Reactivity of polynuclear palladium carboxylate complexes towards acetonitrile: synthesis and X-ray study of Pd2(C6H4-o-C( [[double bond, length as m-dash]] NH)CH3)2(CH3CO2)2 and Pd5(CH3C( [[double bond, length as m-dash]] N)OC( [[double bond, length as Reactivity of polynuclear palladium carboxylate complexes towards acetonitrile: synthesis and X-ray study of Pd2(C6H4-o-C( [[double bond, length as m-dash]] NH)CH3)2(CH3CO2)2 and Pd5(CH3C( [[double bond, length as m-dash]] N)OC( [[double bond, length as](https://pubs.rsc.org/image/article/2013/dt/c2dt32407e/c2dt32407e-s5.gif)
Reactivity of polynuclear palladium carboxylate complexes towards acetonitrile: synthesis and X-ray study of Pd2(C6H4-o-C( [[double bond, length as m-dash]] NH)CH3)2(CH3CO2)2 and Pd5(CH3C( [[double bond, length as m-dash]] N)OC( [[double bond, length as
![Reactivity of polynuclear palladium carboxylate complexes towards acetonitrile: synthesis and X-ray study of Pd2(C6H4-o-C( [[double bond, length as m-dash]] NH)CH3)2(CH3CO2)2 and Pd5(CH3C( [[double bond, length as m-dash]] N)OC( [[double bond, length as Reactivity of polynuclear palladium carboxylate complexes towards acetonitrile: synthesis and X-ray study of Pd2(C6H4-o-C( [[double bond, length as m-dash]] NH)CH3)2(CH3CO2)2 and Pd5(CH3C( [[double bond, length as m-dash]] N)OC( [[double bond, length as](https://pubs.rsc.org/image/article/2013/dt/c2dt32407e/c2dt32407e-f2.gif)
Reactivity of polynuclear palladium carboxylate complexes towards acetonitrile: synthesis and X-ray study of Pd2(C6H4-o-C( [[double bond, length as m-dash]] NH)CH3)2(CH3CO2)2 and Pd5(CH3C( [[double bond, length as m-dash]] N)OC( [[double bond, length as

Recent Progress in the Use of Pd-Catalyzed C-C Cross-Coupling Reactions in the Synthesis of Pharmaceutical Compounds

Understanding Palladium Acetate from a User Perspective - Carole - 2016 - Chemistry – A European Journal - Wiley Online Library

Auxiliary-assisted palladium-catalyzed arylation and alkylation of sp2 and sp3 carbon-hydrogen bonds. - Abstract - Europe PMC

Transition metal-catalyzed cross-coupling methodologies for the engineering of small molecules with applications in organic electronics and photovoltaics - ScienceDirect

Understanding Palladium Acetate from a User Perspective - Carole - 2016 - Chemistry – A European Journal - Wiley Online Library

α,β-Dehydrogenation of esters with free OH and NH functionalities via allyl- palladium catalysis - ScienceDirect

Colorimetric and fluorescent probe for real-time detection of palladium (II) ion in aqueous medium and live cell imaging - ScienceDirect
![Reactivity of polynuclear palladium carboxylate complexes towards acetonitrile: synthesis and X-ray study of Pd2(C6H4-o-C( [[double bond, length as m-dash]] NH)CH3)2(CH3CO2)2 and Pd5(CH3C( [[double bond, length as m-dash]] N)OC( [[double bond, length as Reactivity of polynuclear palladium carboxylate complexes towards acetonitrile: synthesis and X-ray study of Pd2(C6H4-o-C( [[double bond, length as m-dash]] NH)CH3)2(CH3CO2)2 and Pd5(CH3C( [[double bond, length as m-dash]] N)OC( [[double bond, length as](https://pubs.rsc.org/en/Content/Image/GA/C2DT32407E)




.jpg)


