
Cost Effectiveness Modelling for Health Technology Assessment: A Practical Course: 9783319157436: Medicine & Health Science Books @ Amazon.com

Regulatory and health technology assessment advice on postlicensing and postlaunch evidence generation is a foundation for lifecycle data collection for medicines - Moseley - 2020 - British Journal of Clinical Pharmacology - Wiley Online Library

The domains of the HTA Core Model. HTA, health technology assessment;... | Download Scientific Diagram

PDF) HEALTH TECHNOLOGY ASSESSMENT METHODS GUIDELINES FOR MEDICAL DEVICES: HOW CAN WE ADDRESS THE GAPS? THE INTERNATIONAL FEDERATION OF MEDICAL AND BIOLOGICAL ENGINEERING PERSPECTIVE

Health Technology Assessment and Health Policy Today: A Multifaceted View of their Unstable Crossroads | Juan E. del Llano-Señarís | Springer
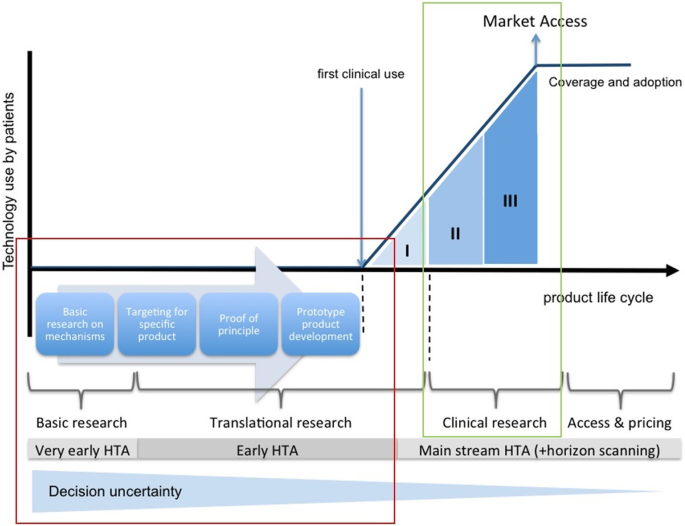
Defining the clinician's role in early health technology assessment during medical device innovation – a systematic review | BMC Health Services Research | Full Text
Bridging the Gap: Pathways for Regulatory and Health Technology Assessment of Histology Independent Therapies | OHE

Translating HTA into Policy and Practice: Setting a Path to Self Reliance | Management Sciences for Health

Sustainability | Free Full-Text | From Health Technology Assessment to Health Technology Sustainability | HTML

HTAi på Twitter: "There's no time like the present to start thinking about our next Annual Meeting! Have you started planning for #HTAi2019Cologne? Check out the great video of our live infographic
![PDF] HEALTH TECHNOLOGY ASSESSMENT METHODS GUIDELINES FOR MEDICAL DEVICES: HOW CAN WE ADDRESS THE GAPS? THE INTERNATIONAL FEDERATION OF MEDICAL AND BIOLOGICAL ENGINEERING PERSPECTIVE. | Semantic Scholar PDF] HEALTH TECHNOLOGY ASSESSMENT METHODS GUIDELINES FOR MEDICAL DEVICES: HOW CAN WE ADDRESS THE GAPS? THE INTERNATIONAL FEDERATION OF MEDICAL AND BIOLOGICAL ENGINEERING PERSPECTIVE. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/ddf4b1b3671fe11e18f7a0a1635ca18283a2afb0/11-Table3-1.png)
PDF] HEALTH TECHNOLOGY ASSESSMENT METHODS GUIDELINES FOR MEDICAL DEVICES: HOW CAN WE ADDRESS THE GAPS? THE INTERNATIONAL FEDERATION OF MEDICAL AND BIOLOGICAL ENGINEERING PERSPECTIVE. | Semantic Scholar

HEALTH TECHNOLOGY ASSESSMENT METHODS GUIDELINES FOR MEDICAL DEVICES: HOW CAN WE ADDRESS THE GAPS? THE INTERNATIONAL FEDERATION OF MEDICAL AND BIOLOGICAL ENGINEERING PERSPECTIVE

Market Access in Europe: Bridging Regulatory and Health Technology Assessment (HTA) Gaps | Voisin Consulting Life Sciences
![PDF] Using health technology assessment to identify research gaps: an unexploited resource for increasing the value of clinical research. | Semantic Scholar PDF] Using health technology assessment to identify research gaps: an unexploited resource for increasing the value of clinical research. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/f7aeefd68826158d422d8dda8ce2fc5ca37b0d91/9-Table2-1.png)





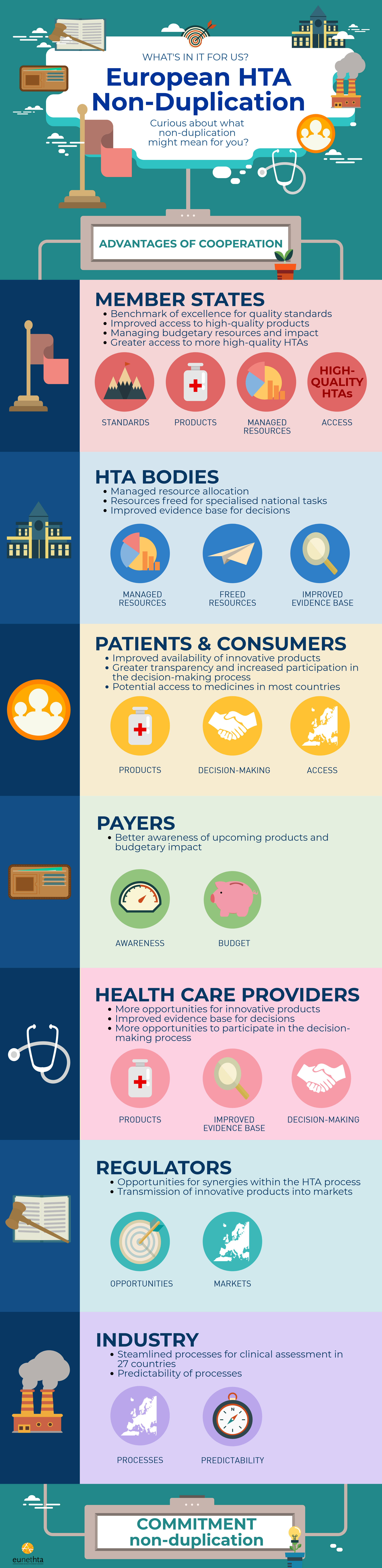



![Full text] The role of health technology assessment bodies in shaping drug develo | DDDT Full text] The role of health technology assessment bodies in shaping drug develo | DDDT](https://www.dovepress.com/cr_data/article_fulltext/s49000/49935/img/DDDT-49935-T01.png)